Cell and Its Environment
The environment of a cell refers to what surrounds it. The cell environment of a unicellular organism includes air, water, soil, or parts of a living organism (in the case of a parasite). In multicellular organisms, the cell environment includes other cells and some tissue fluid. For a cell to survive and function properly, it must exchange materials with its environment in the following ways:
Diffusion
Diffusion is the process by which molecules or ions of a substance (solid, liquid, or gas) move from a region of high concentration to a region of low concentration. The difference in concentration before diffusion occurs is called the concentration gradient.
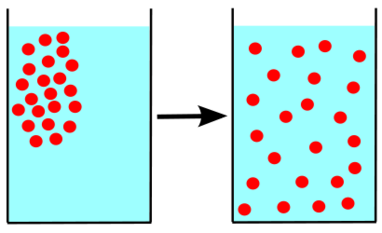 Credit: Wikipedia
Credit: Wikipedia
Factors That Affect the Rate of Diffusion
- State of Matter: Gases diffuse faster than liquids because gaseous molecules can move freely.
- Molecular Size: Smaller molecules diffuse faster.
- Difference in Concentration: A greater difference in concentration results in a faster rate of diffusion.
- Temperature: Higher temperatures increase the rate of diffusion.
Importance of Diffusion to Living Organisms
- The movement of carbon dioxide through the stomata of leaves during photosynthesis.
- Gaseous exchange in the lungs of mammals.
- Intake of oxygen or nutrients from mother to fetus (embryo) through the placenta.
- Movement of digested and soluble food from the villi of the small intestine to the bloodstream.
- Movement of oxygen into leaves during respiration.
- Water vapor leaving leaves during transpiration.
- Gaseous exchange in unicellular organisms occurs by diffusion.
- In plants, food particles from phloem and water from xylem are transferred through diffusion.
Osmosis
Osmosis is the movement of water molecules from a dilute solution into a more concentrated solution through a semi-permeable membrane. A permeable membrane allows molecules to pass through freely, while a selectively permeable membrane only allows certain molecules to pass. Osmosis occurs when a semi-permeable membrane separates weak and strong solutions.
Types of Solutions in Osmosis
- Hypotonic Solution: This solution has a lower concentration than its environment. There is a net movement of water molecules from the surrounding fluid into the cells (endosmosis).
- Isotonic Solution: A solution with the same concentration as its environment, resulting in no net movement of water molecules in or out of the cells.
- Hypertonic Solution: This solution has a higher concentration than its environment, causing a net movement of water molecules out of the cell (exosmosis), leading to cell shrinkage.
Importance of Osmosis
- Regulates the concentrations of salt and water in blood and other body fluids in animals.
- Enables the absorption of water from the soil solution to plants through root hairs.
- Helps control the opening and closing of stomata.
- Aids in the reabsorption of water from kidney tubules into the blood.
- Provides turgidity to plant cells, giving support.
Osmotic Pressure
This is the pressure created when water moves across a membrane into a solution of higher concentration. It is the force that draws water into the cell.
Plasmolysis
Plasmolysis is the outward movement of water from a living cell when placed in a hypertonic solution, causing the cell to shrink and become dehydrated. In plant cells, this results in exosmosis, where water moves out of the cell into the surrounding fluid, leading to the shrinking of the vacuole and pulling the cytoplasm away from the cell wall. Plasmolysis can eventually cause wilting or death of the plant.
Hemolysis
Hemolysis is the rupturing of red blood cells resulting from excessive water intake when placed in a hypotonic solution. Red blood cells and blood plasma are normally isotonic. If the concentration of blood plasma falls, endosmosis occurs, causing water to continuously enter the cells. When fully stretched, the cells burst.
Turgidity
Turgidity is the state in which a cell has absorbed a large quantity of water and becomes fully stretched. The cell is said to be turgid due to turgor pressure. Turgidity is observed in plants, making them erect and supporting stems, leaves, and flowers.
Flaccidity
Flaccidity occurs when plants lose water to their surroundings faster than they can absorb it. When a plant loses more water than it absorbs, it becomes flaccid. Continuous water loss can lead to the death of the plant.
Crenation
Crenation refers to the abnormal notching or shrinking of cells caused by water loss through osmosis. Under normal conditions, cells are surrounded by an isotonic solution, where the concentrations of solutes and water are balanced both inside and outside the cells. This balance allows cells to maintain their shape, as water moves in and out at a steady rate, keeping osmotic pressure stable.
When cells are placed in a hypertonic solution with a higher solute concentration outside the cell, water diffuses out through the cell membrane. This water loss causes the cells to shrink and develop notched or spiked edges, a process known as crenation.
Red Blood Cell Crenation
Red blood cells are especially susceptible to crenation due to changes in ionic concentration in the blood or abnormalities in the cell membrane. This disrupts their ability to maintain an isotonic state. Crenated red blood cells can be classified as:
- Echinocytes: Cells with evenly spaced projections.
- Acanthocytes: Cells with irregular, spiky projections.
Crenation in Food Pickling
Crenation can also be observed during food pickling. When vegetables like cucumbers are placed in acidic pickling solutions, water diffuses out of the cells, causing them to shrink. This shrinkage is a result of the crenation process.
Active Transport
Active transport is a type of transport that does not rely on a concentration gradient. It is a method by which particles can cross membranes even against a concentration gradient using energy (e.g., Na+K+ transport and the movement of protein molecules across cell membranes).